How to Convert Climate-Changing Carbon Dioxide Into Plastics and Other Products
Rutgers scientists develop green chemistry based on a natural process
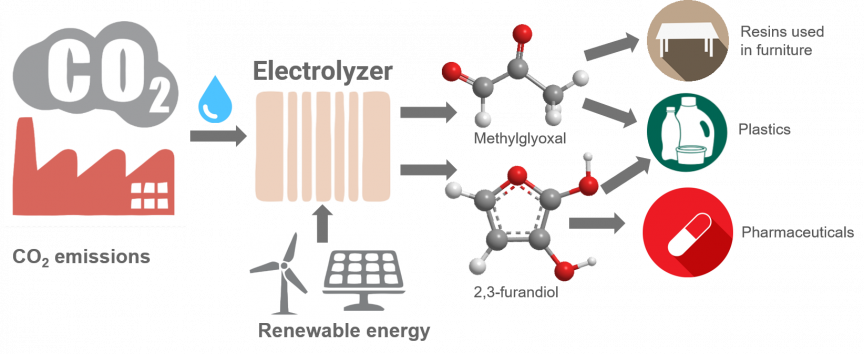
Rutgers scientists have developed catalysts that can convert carbon dioxide – the main cause of global warming – into plastics, fabrics, resins and other products.
The electrocatalysts are the first materials, aside from enzymes, that can turn carbon dioxide and water into carbon building blocks containing one, two, three or four carbon atoms with more than 99 percent efficiency. Two of the products created by the researchers – methylglyoxal (C3) and 2,3-furandiol (C4) – can be used as precursors for plastics, adhesives and pharmaceuticals. Toxic formaldehyde could be replaced by methylglyoxal, which is safer.
The precursors could serve as an alternative to using petroleum for making plastics.
The discovery, based on the chemistry of artificial photosynthesis, is detailed in the journal Energy & Environmental Science.
“Our breakthrough could lead to the conversion of carbon dioxide into valuable products and raw materials in the chemical and pharmaceutical industries,” said study senior author Charles Dismukes, Distinguished Professor in the Department of Chemistry and Chemical Biology and Department of Biochemistry and Microbiology at Rutgers University–New Brunswick. He is also a principal investigator at Rutgers’ Waksman Institute of Microbiology.
Previously, scientists showed that carbon dioxide can be electrochemically converted into methanol, ethanol, methane and ethylene with relatively high yields. But such production is inefficient and too costly to be commercially feasible, according to study lead author Karin Calvinho, a chemistry doctoral student in Rutgers’ School of Graduate Studies.
However, carbon dioxide and water can be electrochemically converted into a wide array of carbon-based products, using five catalysts made of nickel and phosphorus, which are cheap and abundant, she said. The choice of catalyst and other conditions determine how many carbon atoms can be stitched together to make molecules or even generate longer polymers. In general, the longer the carbon chain, the more valuable the product.
Based on their research, the Rutgers scientists earned patents for the electrocatalysts and formed RenewCO₂, a start-up company. The next step is to learn more about the underlying chemical reaction, so it can be used to produce other valuable products such as diols, which are widely used in the polymer industry, or hydrocarbons that can be used as renewable fuels. The Rutgers experts are designing, building and testing electrolyzers for commercial use.